Caring for patients with hemophilia B?
Talk to your patients about BEYOND-9
With no cure for hemophilia B, the BEYOND-9 clinical research study is currently underway to investigate potential long-term treatment options that may benefit both adults and children. Consider BEYOND-9 as an opportunity for your patients with hemophilia B to access investigational treatments.
BEYOND-9 is a first-in human (FIH) phase 1/2 study to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of an investigational treatment in participants with severe and moderately severe congenital hemophilia B with FIX functional activity ≤ 2%.
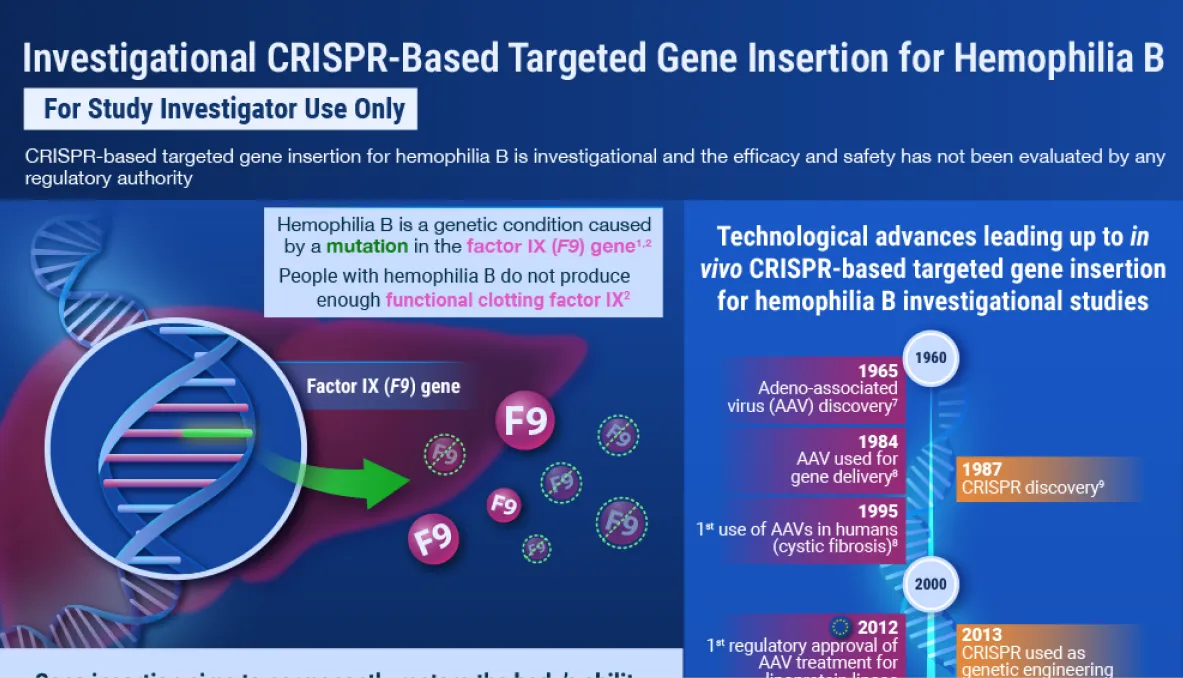
The study treatment is an investigational clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-based gene insertion therapy. It is comprised of 2 components to be administered as a one-time, sequential systemic intravenous infusion.
Component 1 contains a promoter-less, F9 DNA gene encoding wild-type human FIX protein, delivered using recombinant adeno-associated virus serotype 8 (rAAV8). The F9 DNA template is inserted at the location where the double-strand DNA break (DSB) has occurred and is transcribed under the influence of the endogenous Albumin (ALB) promoter, resulting in wild-type human FIX protein expression and secretion from the liver.
Component 2 uses a modular lipid nanoparticle (LNP) technology to deliver the ALB-targeting single guide RNA (sgRNA) and the mRNA coding for the RNA-guided Cas9 endonuclease to hepatocytes, enabling introduction of a site-specific DSB in the first intron of the ALB locus.
Learn more
Patients are expected to participate in this study for up to approximately 2 years and 8 months. Participation may last longer (up to 4 years and 2 months) depending on time spent in the lead-in period.
Participants will be reimbursed for reasonable travel expenses to and from study visits, and medical care related to their participation, including the study treatment, will be provided at no cost.
How can you help?
BEYOND-9 is enrolling up to approximately 120 people across approximately 10 countries worldwide. The success of BEYOND-9 depends on physicians to refer potential study participants. Please review the key study criteria below to help identify any potential candidates.
Key inclusion criteria:
- Male (≥18 years of age)
NOTE: In the future, pediatrics are planned to be included in the clinical trial (between 2 and 17 years of age) - Confirmed diagnosis of severe or moderately severe hemophilia B with medical history of FIX functional activity ≤2% or <0.02 IU/mL
- Currently taking FIX prophylaxis and previous experience with FIX therapy: ≥150 documented exposure days to a FIX protein product such as recombinant, plasma-derived, or extended half-life FIX product
- Participation in the lead-in period of this interventional study OR a separate lead-in study (R000-HEMB-2187) for at least 6 months for collection of ABR data while taking FIX prophylaxis
NOTE: The first 3 participants included in Part 1 of the study are not required to participate in the lead-in period
Key exclusion criteria:
- History of FIX inhibitor (clinical or laboratory-based assessment) on 2 or more occasions
- Bethesda inhibitor titer greater than the upper limit of normal (ULN) at screening
- Detectable pre-existing antibodies to the AAV8 capsid; as measured by ELISA at pre-screening (or final lead-in visit, if applicable)
- A participant is not eligible if any of the following pre-existing diagnoses, which are indicative of significant underlying liver disease, are documented:
- Cholestatic liver disease
- Liver cirrhosis
- Portal hypertension
- Splenomegaly
- Hepatic encephalopathy
- Evidence of advanced liver fibrosis suggestive of or equal to METAVIR Stage 3 disease at screening (i.e., liver stiffness measurement ≥ 9 kPa) as measured by transient elastography (FibroScan) at screening or measured within 6 months prior to the screening visit
- Evidence of cirrhosis and/or portal hypertension as assessed by liver ultrasound at screening or measured within 6 months prior to the screening visit
- History of arterial or venous thrombo-embolic events including but not limited to non-hemorrhagic stroke, myocardial infarction, arterial embolus, deep vein thrombosis, pulmonary embolism, with the exception of catheter-associated thrombosis for which antithrombotic treatment is not currently ongoing
- History of hypersensitivity to corticosteroids or known medical condition that requires chronic administration of corticosteroids
- Previously received any AAV gene-based therapy with a marketed gene therapy or in a clinical trial, or intends to receive approved or investigational AAV-based gene therapy other than the study treatment during the study period
Please note: Other protocol-defined inclusion/exclusion criteria apply.
The study team will check your patient’s full medical history to see if they meet all inclusion criteria and none of the exclusion criteria. Diversity in clinical studies is critical to developing better treatments – help ensure that your patients are represented.
If you have potentially eligible and interested patients, empower them with options and discuss BEYOND-9. You may also contact the nearest study site to refer them.
Find a study site near you!
Your patient’s health and safety are our top priorities. We hope you value the impact of this research and will consider referring potential candidates. With your support, we hope to help patients with hemophilia B.